Our research:
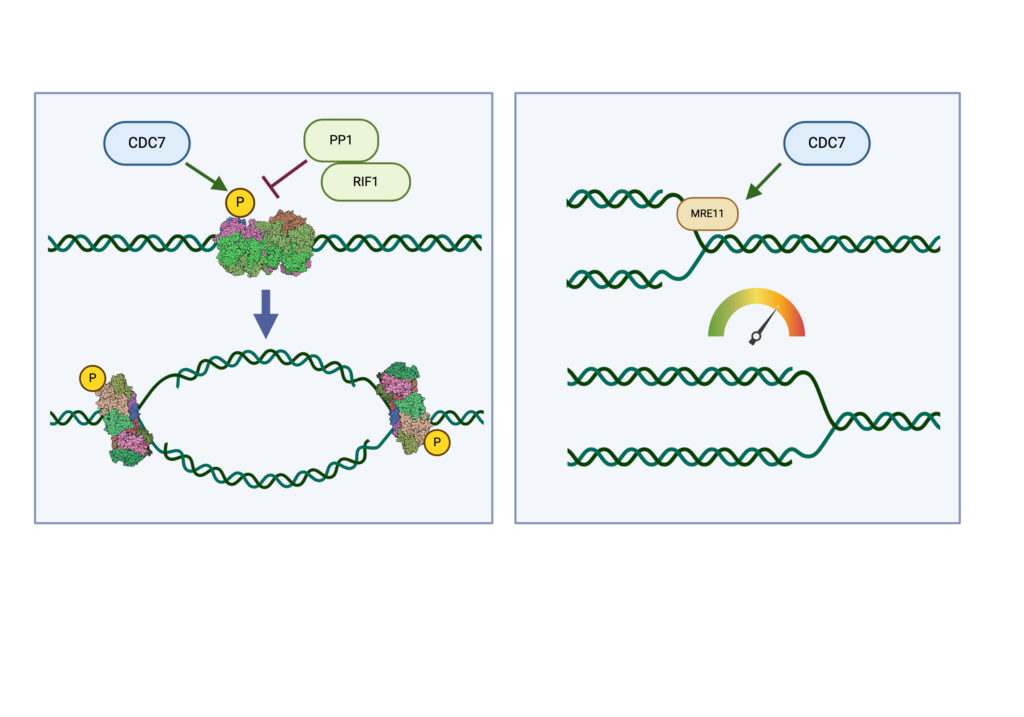
The process of DNA replication is the biggest threat for genome stability in all proliferating cells. Cancer cells in particular are subjected to replication stress after activation of proto-oncogenes into their oncogenic forms or due to deficiency in specific factors required for genome duplication. Thus aberrant DNA replication contributes to initiating and maintaining the cancerous state, while drugs targeting DNA synthesis have potent antitumor activity and are key component of current and novel chemotherapeutic regimens. Our laboratory is studying the mechanisms that regulate genome replication in human cells with particular emphasis on the Cell Division Cycle 7 kinase (CDC7). CDC7 acts as a molecular switch for DNA synthesis at origins of replication and controls the processing of replication forks through the MRE11 nuclease. CDC7 is also thought to participate in several other processes that regulate normal cell cycle progression and chromosome dynamics. We apply modern techniques of Molecular and Cellular Biology as well Chemical Biology and Genomics approaches to reveal how cells duplicate their DNA and to understand cellular responses when this process is perturbed by either intrinsic or extrinsic factors.
Two stories narrated by our team members in 12 minutes:
Consequences of CDC7 inhibition:
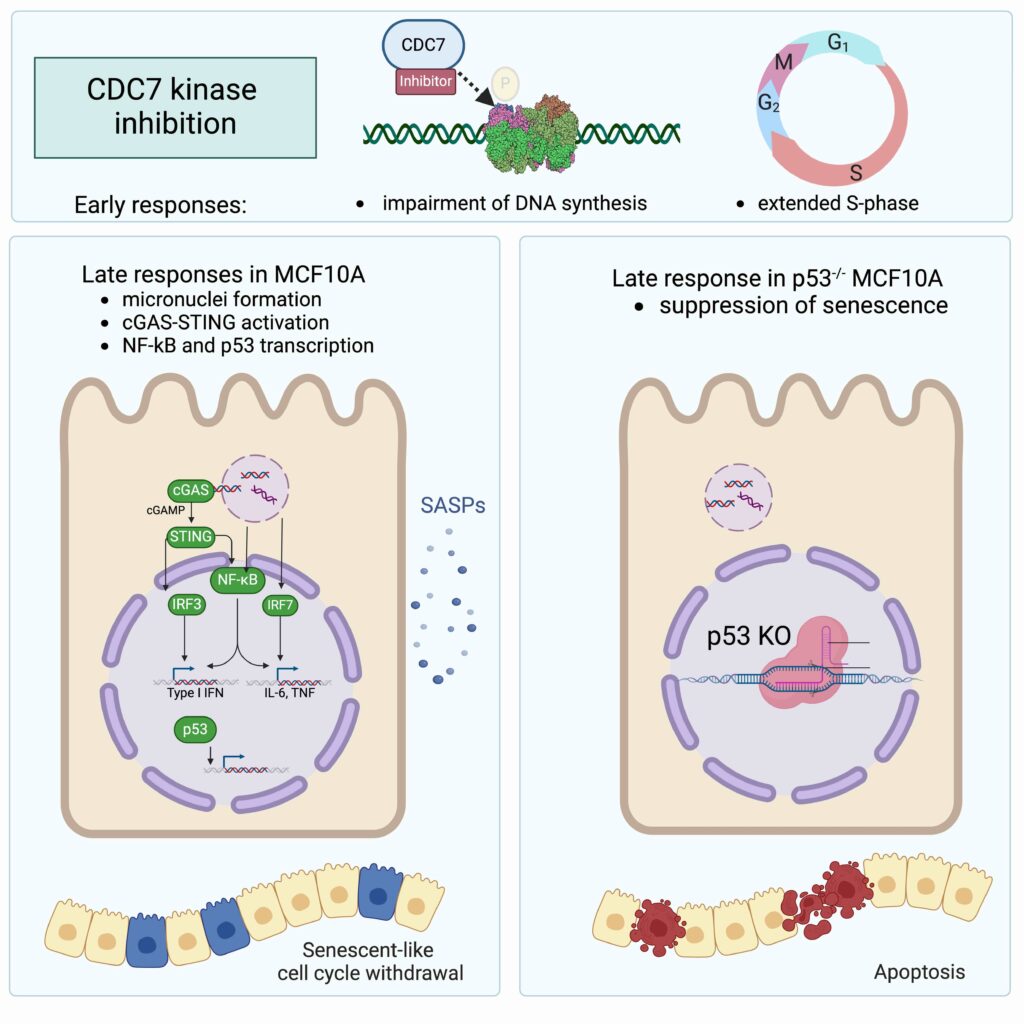
Drugs that block DNA replication prevent cell proliferation, which may result in anticancer activity. The latter is dependent on the drug’s mode of action as well as on cell type-dependent responses to treatment. The inhibition of Cell division cycle 7-related protein kinase (CDC7), a key regulator of DNA replication, decreases the efficiency of origin firing and hampers the restarting of paused replication forks. Here, we show that upon prolonged CDC7 inhibition, breast-derived MCF10A cells progressively withdraw from the cell cycle and enter a reversible senescent-like state. This is characterised by the rewiring of the transcriptional programme with the induction of cytokine and chemokine expression and correlates with the accumulation of Cyclic GMP-AMP synthase (cGAS)-positive micronuclei. Importantly, cell fate depends on Cellular tumour antigen p53 (p53) function as cells no longer enter senescence but are funnelled into apoptosis upon p53 knockout. This work uncovers key features of the secondary response to CDC7 inhibitors, which could aid the development of these compounds as anticancer drugs.
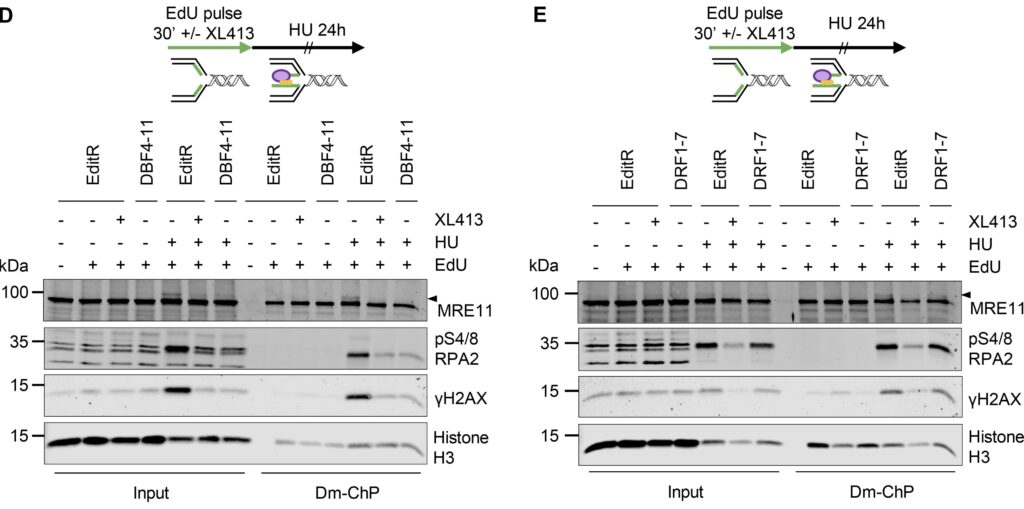
Two regulatory subunits for CDC7:
CDC7 kinase is crucial for DNA replication initiation and is involved in fork processing and replication stress response. Human CDC7 requires the binding of either DBF4 or DRF1 for its activity. However, it is unclear whether the two regulatory subunits target CDC7 to a specific set of substrates, thus having different biological functions, or if they act redundantly. Using genome editing technology, we generated isogenic cell lines deficient in either DBF4 or DRF1: these cells are viable but present signs of genomic instability, indicating that both can independently support CDC7 for bulk DNA replication. Nonetheless, DBF4-deficient cells show altered replication efficiency, partial deficiency in MCM helicase phosphorylation, and alterations in the replication timing of discrete genomic regions. Notably, we find that CDC7 function at replication forks is entirely dependent on DBF4 and not on DRF1. Thus, DBF4 is the primary regulator of CDC7 activity, mediating most of its functions in unperturbed DNA replication and upon replication interference.